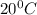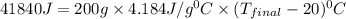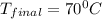Chemistry, 12.06.2020 21:57 jalaholmes2027
If 200. g of water at 20°C absorbs 41 840 J of energy, what will its final temperature be? (Specific Heat of water is 4.184 J/g*C)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 13:00
What type of reaction is represented by the following example? 2co2 (g) + 4h2o (l) + 1452 kj 2ch3oh (l) (g) + 3o2 (g) exothermic endothermic
Answers: 1

Chemistry, 21.06.2019 19:30
If the root word engage means “to connect with something,” what does the word disengage mean in the following sentence? he disengaged the gears by stepping on the clutch pedal.a.added more engine powerb.activated a connection to the pedalc.stalled the engined.released a connection to the pedal
Answers: 1

Chemistry, 22.06.2019 04:40
In which environment would primary succession occur? a forest with a few remaining trees after a recent wildfire an area of exposed rock after a glacier melts away beach that is exposed to the air at low tide an abandoned baseball field in a small town
Answers: 1

Chemistry, 22.06.2019 10:10
When water dissociates, each water molecule splits into a hydroxide ion and a) h 3 o + b) a hydrogen atom c) a hydrogen ion d) h 2 o e) oh —
Answers: 2
You know the right answer?
If 200. g of water at 20°C absorbs 41 840 J of energy, what will its final temperature be? (Specific...
Questions

Advanced Placement (AP), 05.03.2021 20:20




Mathematics, 05.03.2021 20:20

Mathematics, 05.03.2021 20:20

Biology, 05.03.2021 20:20

English, 05.03.2021 20:20

Mathematics, 05.03.2021 20:20

Mathematics, 05.03.2021 20:20

Mathematics, 05.03.2021 20:20




Mathematics, 05.03.2021 20:20

English, 05.03.2021 20:20

Social Studies, 05.03.2021 20:20

Physics, 05.03.2021 20:20

Biology, 05.03.2021 20:20


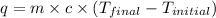
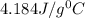
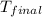 = final temperature =?
= final temperature =?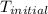 = initial temperature =
= initial temperature =