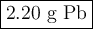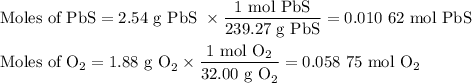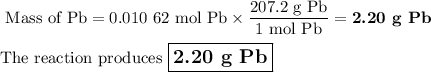I NEED HELP PLEASE, THANKS! :) To obtain pure lead, lead (II) sulfide is burned in an atmosphere of pure oxygen. The products of the reaction are lead and sulfur trioxide (SO3). Write a balanced chemical equation for this process. How many grams of lead will be produced if 2.54 grams of PbS is burned with 1.88 g of O2? Express your answer to the correct number of significant figures.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 12:50
The number at the end of an isotope’s name is the number.
Answers: 1

Chemistry, 22.06.2019 16:00
No copying 15 pts how does a free-body diagram tell you about the net force on an object?
Answers: 2

Chemistry, 23.06.2019 04:00
How much energy is required to vaporize 2 kg of copper? a 4730 kj b 207kj c 9460 kj d 414kj
Answers: 1
You know the right answer?
I NEED HELP PLEASE, THANKS! :)
To obtain pure lead, lead (II) sulfide is burned in an atmosphere of...
Questions


Mathematics, 10.03.2021 14:20

Mathematics, 10.03.2021 14:20



Chemistry, 10.03.2021 14:20

Mathematics, 10.03.2021 14:20

Mathematics, 10.03.2021 14:20


Mathematics, 10.03.2021 14:20


History, 10.03.2021 14:20



Mathematics, 10.03.2021 14:20

Business, 10.03.2021 14:20

Geography, 10.03.2021 14:20

Mathematics, 10.03.2021 14:20


Mathematics, 10.03.2021 14:20