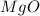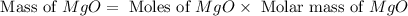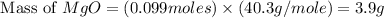Chemistry, 13.06.2020 03:57 hardwick744
During a synthesis reaction, 2.4 grams of magnesium reacted with 8.0 grams of oxygen. What is the maximum amount of magnesium oxide that can be produced during the reaction?
Mg + O2 → MgO
2.1 grams
2.8 grams
3.6 grams
3.9 grams

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Design techniques and materials that reduce the negative environmental impact of a structure are referred to as
Answers: 2

Chemistry, 23.06.2019 00:00
Before it was launched, a helium-filled balloon had a pressure of 201 kpa at a temperature of 27°c. at an altitude of 15,000 m, the pressure had decreased to 2.5 kpa and the temperature had dropped to -14 °c. the volume of the balloon increased to 59.3 m3. what is the original volume of the balloon? 13 m3 0.85 m3 0.077 m3 1.17 m3
Answers: 3

Chemistry, 23.06.2019 04:40
Equal numbers of moles of he(g), ar(g), and ne(g) are placed in a glass vessel at room temperature. if the vessel has a pinhole-sized leak, which of the following will be true regarding the relative values of the partial pressures of the gases remaining in the vessel after some of the gas mixture has effused?
Answers: 1

Chemistry, 23.06.2019 06:00
In an exothermic reaction at equilibrium, what is the effect of lowering the temperature? a. the reaction makes more products. b. the reaction makes more reactants. c. the reaction is unchanged.
Answers: 1
You know the right answer?
During a synthesis reaction, 2.4 grams of magnesium reacted with 8.0 grams of oxygen. What is the ma...
Questions


Mathematics, 31.01.2020 00:46


Spanish, 31.01.2020 00:46

Mathematics, 31.01.2020 00:46




Social Studies, 31.01.2020 00:46


Mathematics, 31.01.2020 00:46

Biology, 31.01.2020 00:46

Mathematics, 31.01.2020 00:46

Mathematics, 31.01.2020 00:46

Mathematics, 31.01.2020 00:46



Mathematics, 31.01.2020 00:46

History, 31.01.2020 00:46

Social Studies, 31.01.2020 00:46

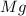 = 2.4 g
= 2.4 g
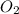 = 8.0 g
= 8.0 g
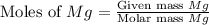
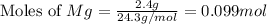
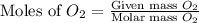
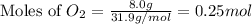
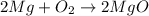
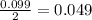 moles of
moles of