

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Type the correct answer in the box. spell all words correctly.what is the correct term for living the most sustainable life you can within your current circumstances? when your are being as sustainable as you can within your current lifestyle, you are said to be sustainability.
Answers: 3

Chemistry, 23.06.2019 00:30
What are the advantages of using the metric system? designed as a decimal system making conversions simpler more accurate system of measurement has prefixes that correspond to an amount to use with all base units used by the entire scientific community
Answers: 2

Chemistry, 23.06.2019 02:00
The plant food contains nh4)3po4 what tests would you run to verify the presence of the nh4 ion and the po4 ion
Answers: 2

You know the right answer?
Determine the temperature of 2.49mol of a gas contained in a 1.00L vessel at a pressure of 1.41atm....
Questions

Mathematics, 07.07.2021 18:40


Mathematics, 07.07.2021 18:40






Chemistry, 07.07.2021 18:40

English, 07.07.2021 18:40




Business, 07.07.2021 18:50



Mathematics, 07.07.2021 18:50

Mathematics, 07.07.2021 18:50

World Languages, 07.07.2021 18:50

Business, 07.07.2021 18:50

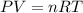
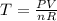
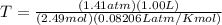
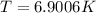
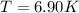 .
.

