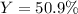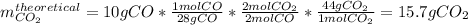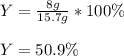Chemistry, 13.06.2020 05:57 michaelwarren8728
Consider the reaction 2 CO + O2 → 2 CO2 .What is the percent yield of carbon dioxide
(MW = 44 g/mol) if the reaction of 10 g of carbon monoxide (MW = 28 g/mol) with
excess O2 produces 8 g of carbon dioxide? Answer in units of %.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 13:10
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1

Chemistry, 22.06.2019 15:30
Draw the lewis dot structure for each of the following polyatomic ions
Answers: 1

Chemistry, 22.06.2019 20:00
Glucose (c6h12o6) is an important biological molecule. (round the answer to nearest hundredth.) what is the percent by mass of carbon in glucose?
Answers: 2

Chemistry, 22.06.2019 22:00
All of the following are homogeneous mixtures except a) sugar dissolved in water. b) orange juice. c) coffee with cream. d) household vinegar. e) apple juice
Answers: 1
You know the right answer?
Consider the reaction 2 CO + O2 → 2 CO2 .What is the percent yield of carbon dioxide
(MW = 44 g/mol...
Questions

Computers and Technology, 22.08.2020 21:01




Mathematics, 22.08.2020 21:01



Biology, 22.08.2020 21:01



Mathematics, 22.08.2020 21:01


Chemistry, 22.08.2020 21:01



History, 22.08.2020 21:01

Mathematics, 22.08.2020 21:01


Mathematics, 22.08.2020 21:01