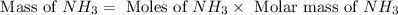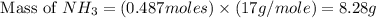Chemistry, 13.06.2020 14:57 snlawson9053
1.46 g H2 is allowed to react with 10.5 g N2, producing 2.72 g NH3. What is the theoretical yield in grams for this reaction under the given conditions? Express your answer to three significant figures and include the appropriate units.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 21:50
28. which is not a reason that water is used to store spent fuel rods from nuclear power plants? water increases the speed of the chain reaction in the fuel rods. water protects nuclear power plant workers from the high temperature and radiation of the fuel rods. water acts as a radiation shield to reduce the radiation levels. water cools the spent rods. salts action
Answers: 1

Chemistry, 23.06.2019 00:10
In as 1°, 2°, 3°, or 4°. be to . : °b: °c: °d: ° : °b: °c: °d: ° : °b: °c: °d: °e: °f: °g: °h: ° : °b: °c: °d: °e: °f: °g: °h: °i: °
Answers: 3

Chemistry, 23.06.2019 04:10
An unknown substance has been shown to have metallic bonds. which of the following is most likely a property of this substance? a. low conductivity b. low boiling point c. high malleability d. high solubility in water
Answers: 2

Chemistry, 23.06.2019 15:30
Can someone me with these problems? see the attachment, .
Answers: 1
You know the right answer?
1.46 g H2 is allowed to react with 10.5 g N2, producing 2.72 g NH3. What is the theoretical yield in...
Questions

Mathematics, 07.01.2021 01:00





Mathematics, 07.01.2021 01:00


Chemistry, 07.01.2021 01:00




History, 07.01.2021 01:00

Biology, 07.01.2021 01:00

Mathematics, 07.01.2021 01:00

Mathematics, 07.01.2021 01:00

Mathematics, 07.01.2021 01:00


Mathematics, 07.01.2021 01:00

Social Studies, 07.01.2021 01:00

Mathematics, 07.01.2021 01:00

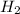 = 1.46 g
= 1.46 g
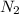 = 10.5 g
= 10.5 g
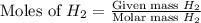
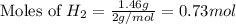
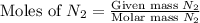
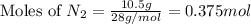
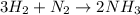
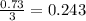 moles of
moles of 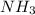
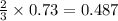 mole of
mole of