I NEED HELP PLEASE, THANKS!
Ammonia (NH3) is an example of a Brønsted-Lowry Base.
-Define the...

Chemistry, 13.06.2020 09:57 hmskevinjacobo5471
I NEED HELP PLEASE, THANKS!
Ammonia (NH3) is an example of a Brønsted-Lowry Base.
-Define the Brønsted-Lowry acid-base theory.
-What is the pH of an ammonia solution that has a concentration of 0.335 M? The Kb of ammonia is 1.8 × 10^–5.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:00
Write the complete balanced equation for the reaction between lead (iv) oxide (pbo2) and water (h2o).
Answers: 1

Chemistry, 21.06.2019 20:30
If 10.g of agno3 is available, what volume of 0.25 m agno3 can be prepared
Answers: 1

Chemistry, 22.06.2019 12:00
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1

You know the right answer?
Questions



Biology, 05.04.2021 23:00

Mathematics, 05.04.2021 23:00






Mathematics, 05.04.2021 23:00


Business, 05.04.2021 23:00


History, 05.04.2021 23:00


Mathematics, 05.04.2021 23:00


History, 05.04.2021 23:00


English, 05.04.2021 23:00

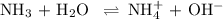
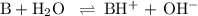
![\rm K_{\text{b}} = \dfrac{\text{[BH}^{+}]\text{[OH}^{-}]}{\text{[B]}} = 1.8 \times 10^{-5}\\\\\dfrac{x^{2}}{0.335 - x} = 1.8 \times 10^{-5}](/tpl/images/0685/0322/975fe.png)
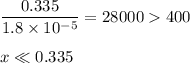
![\dfrac{x^{2}}{0.335} = 1.8 \times 10^{-5}\\\\x^{2} = 0.335 \times 1.8 \times 10^{-5}\\x^{2} = 6.03 \times 10^{-6}\\x = \sqrt{6.03 \times 10^{-6}}\\x = \text{[OH]}^{-} = \mathbf{2.46 \times 10^{-3}} \textbf{ mol/L}](/tpl/images/0685/0322/0aa42.png)
![\text{pOH} = -\log \text{[OH}^{-}] = -\log(2.46 \times 10^{-3}) = 2.61](/tpl/images/0685/0322/acbc3.png)


