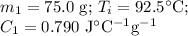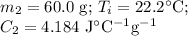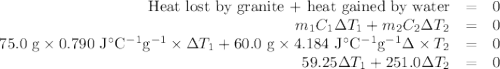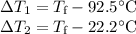Chemistry, 12.06.2020 21:57 dinosaur10
A 75.0 g sample of granite initially at 92.5oC is immersed into 60.0 g of water initially at 22.2oC. Determine the final temperature (in oC) when they reach thermal equilibrium. Assume no heat loss to the surroundings. Specific heats: granite = 0.790 J/(goC), water = 4.184 J/goC

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2


Chemistry, 22.06.2019 17:00
Which statement is true about a catalyst? a: a catalyst decreases the rate of the reaction. b. a catalyst is consumed during a chemical reaction. c. a catalyst lowers the activation energy of a reaction. d. a catalyst increases the reactant concentration during a reaction.
Answers: 1

Chemistry, 23.06.2019 04:40
6) (a) calculate the absorbance of the solution if its concentration is 0.0278 m and its molar extinction coefficient is 35.9 l/(mol cm). the depth of the cell is 5 mm. (b) what is the %t? (7) calculate the absorbance of the solution if the transmitted light intensity is 70% of the initial light beam intensity
Answers: 1
You know the right answer?
A 75.0 g sample of granite initially at 92.5oC is immersed into 60.0 g of water initially at 22.2oC....
Questions

Mathematics, 24.12.2019 19:31


History, 24.12.2019 19:31


Mathematics, 24.12.2019 19:31





Mathematics, 24.12.2019 19:31

German, 24.12.2019 19:31


History, 24.12.2019 19:31


History, 24.12.2019 19:31