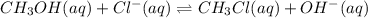Chemistry, 13.06.2020 22:57 donaji1024perez
Write the equilibrium constant expression for this reaction: CH3OH (aq)+Cl-(aq)-> CH3Cl(aq)+OH-(aq)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Calculate the change in entropy if br2(l) is converted to br2(g). s° for br2(l) = 152.2 j/(mol•k) s° for br2(g) = 245.5 j/(mol•k) s° for br(g) = 175.0 j/(mol•k)
Answers: 3

Chemistry, 21.06.2019 22:30
Each of the following compounds contains a metal that can exhibit more than one ionic charge. provide systematic names for each of these compounds. (a) cr(clo3)6 (b) mo(cn)6 (c) cr2(so3)3 (d) v(clo2)2 (e) v(cn)5 (f) os(clo2)4
Answers: 3


Chemistry, 22.06.2019 12:00
What does a complete balanced chemical equation include? a. exothermic coefficients b. endothermic coefficients c. valence electrons d. molar coefficients
Answers: 1
You know the right answer?
Write the equilibrium constant expression for this reaction: CH3OH (aq)+Cl-(aq)-> CH3Cl(aq)+OH-(a...
Questions





History, 18.02.2020 20:58

History, 18.02.2020 20:58



History, 18.02.2020 20:58







Mathematics, 18.02.2020 20:59




![K_c=\frac{[CH_3Cl]\times [OH^-]}{[CH_3OH]\times [Cl^-]}](/tpl/images/0685/4559/fd6a4.png)