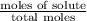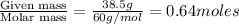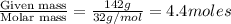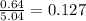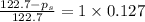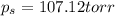Chemistry, 13.06.2020 23:57 reginaldlegette
Calculate the vapor pressure (in torr) at 298 K in a solution prepared by dissolving 38.5 g of the non-volatile non-electrolye urea {CO(NH2)2} in 142 g of methanol. The vapor pressure of methanol at 298 K is 122.7 torr. Give your answer to 2 decimal places.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:10
Why is the vapor pressure of a warm lake higher than the vapor pressure of a cold lake? o a. warm water has a greater heat of vaporization. ob. warm water evaporates more quickly. cool water evaporates more quickly. od. cool water has a greater heat of vaporization.
Answers: 1

Chemistry, 22.06.2019 04:00
4. absorption has the highest risk of overdose due to increased potency. a. rectal b. oral c. transdermal d. intranasal
Answers: 2

Chemistry, 22.06.2019 14:30
For the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 4al(s)+3o2(g)→2al2o3(s) a) 1 molal, 1 mol o2 b) 4 molal, 2.6 mol o2 c) 16 molal, 13 mol o2 d) 7.4 molal, 6.5 mol o2
Answers: 3

Chemistry, 22.06.2019 19:20
For a research project, a student decided to test the effect of the lead(ii) ion (pb2+) on the ability of salmon eggs to hatch. this ion was obtainable from the water‐soluble salt, lead(ii) nitrate, which the student decided to make by the following reaction. pbo(s) + 2 hno3(aq) → pb(no3)2(aq) + h2o losses of product for various reasons were expected, and a yield of 86.0% was expected. in order to have 5.00 g of product at this yield, how many grams of pbo should be reacted? (assume that sufficient nitric acid, hno3, would be used.)
Answers: 1
You know the right answer?
Calculate the vapor pressure (in torr) at 298 K in a solution prepared by dissolving 38.5 g of the n...
Questions


Mathematics, 19.05.2021 05:40


Mathematics, 19.05.2021 05:40





Mathematics, 19.05.2021 05:40

Mathematics, 19.05.2021 05:40


Biology, 19.05.2021 05:40

History, 19.05.2021 05:40


Mathematics, 19.05.2021 05:40


Mathematics, 19.05.2021 05:40


History, 19.05.2021 05:40

Mathematics, 19.05.2021 05:40

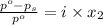
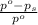 = relative lowering in vapor pressure
= relative lowering in vapor pressure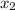 = mole fraction of solute =
= mole fraction of solute =