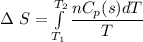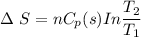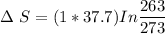Chemistry, 14.06.2020 10:57 nate102201
1 mol super cooled liquid water transformed to solid ice at -10 oC under 1 atm pressure.
a) Calculate entropy change of the system, surrounding and universe. (temperature of the
environment is -10 °C)
b) Make some comments on entropy changes from the obtained data.
Please use the following data for water :
Melting entalpy of ice (ΔHmelting) at 0°C and 1 bar is 6020 J mol-1
.
Cp (H2O (s)) = 37,7 J mol-1 K-1
Cp (H2O (l)) = 75,3 J mol-1 K-1

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:50
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2


Chemistry, 23.06.2019 00:00
How do you determine the percent yield of a chemical reaction
Answers: 1

You know the right answer?
1 mol super cooled liquid water transformed to solid ice at -10 oC under 1 atm pressure.
a) Calcula...
Questions

Social Studies, 04.01.2020 04:31



Engineering, 04.01.2020 04:31

Social Studies, 04.01.2020 04:31

Social Studies, 04.01.2020 04:31

Computers and Technology, 04.01.2020 04:31

Social Studies, 04.01.2020 04:31












Social Studies, 04.01.2020 04:31