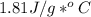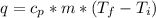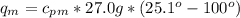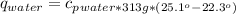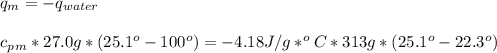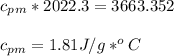Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:00
The reactions shown here can be combined to make the overall reaction c(s) + h2o(g) ⇌ co(g) + h2(g) by reversing some and/or dividing all the coefficients by a number. a. c(s) + o2(g) → co2(g) k=1.363×10^69 b. 2 h2(g) + o2(g) → 2 h2o(g) k=1.389×10^80 c. 2co(g) + o2 (g) → 2 co2(g) k=1.477×10^90
Answers: 1

Chemistry, 22.06.2019 12:30
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1


You know the right answer?
27.0g of an unknown metal at 100 degrees Celcius was dropped into a beaker containing 313g of water...
Questions



Mathematics, 06.02.2021 06:20

Computers and Technology, 06.02.2021 06:20


Arts, 06.02.2021 06:20

History, 06.02.2021 06:20

Engineering, 06.02.2021 06:20

Business, 06.02.2021 06:20



Chemistry, 06.02.2021 06:20


Mathematics, 06.02.2021 06:20

Mathematics, 06.02.2021 06:20


Mathematics, 06.02.2021 06:20

Health, 06.02.2021 06:20


Mathematics, 06.02.2021 06:20