
Chemistry, 14.06.2020 14:57 kenisonpaigebosma
Chlorine dioxide is used as a disinfectant and bleaching agent. In water, it reacts to form chloric acid (HClO3),
according to the following balanced equation:
6 ClO2 + 3 H2O → 5 HClO3 + HCl
If excess ClO2 is mixed with 18.0 mL of H2O (d = 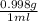 ) how many grams of HClO3 are formed?
) how many grams of HClO3 are formed?
PLEASE DO NOT JUST GIVE THE ANSWER!!! I really would like to learn HOW to do this so please give explanations for each step as you solve it. Thanks!

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
How many moles are in 250 grams of tungsten (w)? * 4.4x10^23 moles 4.2x10^23 moles 0.7 moles 1.4 moles
Answers: 3


Chemistry, 22.06.2019 17:30
What type of organic molecule comprises the majority of a potato?
Answers: 1

Chemistry, 23.06.2019 03:30
If you need to add 27.50ml of a solution, which piece of glassware would you use to deliver this volume and explain how you would determine if the 27.50 ml was measured?
Answers: 1
You know the right answer?
Chlorine dioxide is used as a disinfectant and bleaching agent. In water, it reacts to form chloric...
Questions



Social Studies, 17.10.2020 04:01

Mathematics, 17.10.2020 04:01



Mathematics, 17.10.2020 04:01




Mathematics, 17.10.2020 04:01


Mathematics, 17.10.2020 04:01

Biology, 17.10.2020 04:01



English, 17.10.2020 04:01

Mathematics, 17.10.2020 04:01

Mathematics, 17.10.2020 04:01

English, 17.10.2020 04:01



