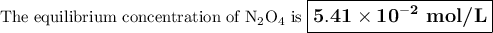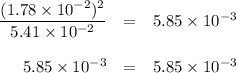Chemistry, 16.06.2020 01:57 Aliciaonfleek
Consider the following equilibrium:
N204(9)
2NO2(9)
Ket
= 5.85 x 10-3
Which statement about this system is true?
Options:
The equilibrium lies to the left.
The equilibrium lies to the right.
If the equilibrium concentration of No, is 1.78 x 10-2 m, the equilibrium concentration of
N204 is
Options:
3.04 M.
1.85 × 10^-6 M.
5.42 × 10^-2 M.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 22:00
Choose all the answers that apply. fluorine (f) has an atomic number of 9 and an atomic weight of 18.99. fluorine has a. 9 protons b. 10 neutrons c. 18 electrons d. an atomic mass of 19 e. at least one isotope
Answers: 1

Chemistry, 23.06.2019 01:00
You wish to prepare a buffer consisting of acetic acid and sodium acetate with a total acetic acetate plus acetate concentration of 250 mm and a ph of 5. what concentrations of acetic acid and sodium acetate should you use
Answers: 1

Chemistry, 23.06.2019 02:00
Now look at the segment of the graph between the two data points marked with black squares. describe how the boiling point and melting point plots behave between these points. be as specific as possible.
Answers: 1
You know the right answer?
Consider the following equilibrium:
N204(9)
2NO2(9)
Ket
= 5.85 x 10-3
Whi...
2NO2(9)
Ket
= 5.85 x 10-3
Whi...
Questions

Mathematics, 03.04.2020 02:35


English, 03.04.2020 02:35

English, 03.04.2020 02:35



Mathematics, 03.04.2020 02:35

Physics, 03.04.2020 02:35

Geography, 03.04.2020 02:35



History, 03.04.2020 02:35



Mathematics, 03.04.2020 02:35

Mathematics, 03.04.2020 02:35




History, 03.04.2020 02:35

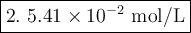
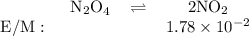
![K_{\text{c}} = \dfrac{\text{[NO$_{2}$]}^{2}}{\text{[N$_{2}$O$_{4}$]}} = \dfrac{(1.78 \times 10^{-2})^{2}}{\text{[N$_{2}$O$_{4}$]}} = 5.85 \times 10^{-3}\\\\\begin{array}{rcl}\\(1.78 \times 10^{-2})^{2}&=&\text{[N$_{2}$O$_{4}$]} \times 5.85 \times 10^{-3}\\\text{[N$_{2}$O$_4$]}&=& \dfrac{(1.78 \times 10^{-2})^{2}}{5.85 \times 10^{-3}}\\\\& = & \mathbf{5.41 \times 10^{-2}}\textbf{ mol/L}\\\end{array}\\](/tpl/images/0686/6303/751d3.png)