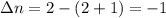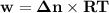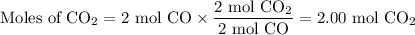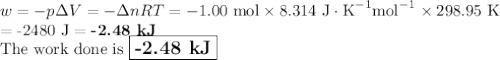Chemistry, 16.06.2020 02:57 estrellaalcantar16
Consider that you have a balloon containing 2.00 moles of CO and 1.00 mole of O2 which is in a room that has a temperature of 25.8oC and a pressure of 1.00 atm. Then, the following reaction occurs inside the balloon to completion: 2 CO(g) + O2(g) -> 2 CO2(g). Calculate the change in work due to the reaction occurring inside the balloon.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Select the correct answer. given: 2libr + ba → babr2 + 2li in this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced? a. 1.18 mol b. 2.37 mol c. 4.73 mol d. 16.4 mol e. 32.9 mol
Answers: 2

Chemistry, 23.06.2019 00:00
#7 how does the structure of amino acids allow them to form a polypeptide? each amino acid has an amino group and a carboxyl group. each amino acid has a hydrogen atom and a carboxyl group. each amino acid has a carboxyl group and an r group. each amino acid has an r group and a hydrogen atom.
Answers: 1

Chemistry, 23.06.2019 01:00
Which fossil fuel is mainly used for heating and cooking? a. electricity b. coal c. petroleum d. natural gas
Answers: 2

You know the right answer?
Consider that you have a balloon containing 2.00 moles of CO and 1.00 mole of O2 which is in a room...
Questions







English, 26.03.2020 17:33






Mathematics, 26.03.2020 17:34

Mathematics, 26.03.2020 17:34

Mathematics, 26.03.2020 17:34

Social Studies, 26.03.2020 17:34


Mathematics, 26.03.2020 17:34


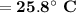
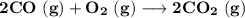
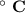 to
to 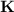 :
: 
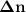 value:
value: