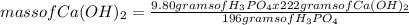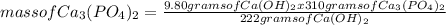Chemistry, 16.06.2020 07:57 perezsamantha3oqr0za
What is the maximum amount of Ca3(PO4)2 that can be prepared from 9.80 g of Ca(OH)2 and 9.80 g of
H3PO4
Ca(OH)2 (s) + H3PO4 (aq)
Ca3(PO4)2 (aq) + H2O (1)
balance the equation 1st.
O 6.80 g
O 15.5 g
O 8.60 g
o 13.7 g
O 10.3 g

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Why do cells appear different in distilled water than they do in 10% salt water?
Answers: 2

Chemistry, 22.06.2019 10:20
In a reaction equation, where are the products located? a.) above the arrow b.) to the right of the arrow c.) to the left of the arrow d.) below the arrow
Answers: 2

Chemistry, 22.06.2019 15:30
How does a large body of water, such as the ocean, influence climate?
Answers: 1

Chemistry, 22.06.2019 19:00
Nan element’s square on the periodic table, the number with the greatest numerical value represents the
Answers: 3
You know the right answer?
What is the maximum amount of Ca3(PO4)2 that can be prepared from 9.80 g of Ca(OH)2 and 9.80 g of
H...
Questions

English, 24.02.2021 20:10


Physics, 24.02.2021 20:10

Mathematics, 24.02.2021 20:10

English, 24.02.2021 20:10

Physics, 24.02.2021 20:10

Chemistry, 24.02.2021 20:10

Spanish, 24.02.2021 20:10

Mathematics, 24.02.2021 20:10

History, 24.02.2021 20:10




Mathematics, 24.02.2021 20:10


History, 24.02.2021 20:10