
Chemistry, 17.06.2020 05:57 maciemessing2
The amino acid glycine is often used as the main ingredient of a buffer in biochemical experiments. The amino group of glycine, which has a pKa of 9.6, can exist either in the protonated form (-NH3+) or as the free base (−NH2), because of the reversible equilibrium:
R-NH3+ left right double arrow R−NH2 + H+
A) In what pH range can glycine be used as an effective buffer due to its amino group?B) In a 0.1 M solution of glycine at pH 9.0, what fraction of glycine has its amino group in the -NH3+ form?C) How much 5 M KOH must be added to 1.0 L of 0.1 M glycine at pH 9.0 to bring its pH to exactly 10.0?D) When 99% of the glycine is in its -NH3+ form, what is the numerical relation between the pH of the solution and the pKa of the amino group?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:00
Which one of the following gases is not an important component of soil?
Answers: 2


Chemistry, 23.06.2019 00:30
The footprints of a dinosaur and the burrow of an ancient shrimp are examples of which kind of fossils
Answers: 2

Chemistry, 23.06.2019 01:30
Which is an example of a highly unstable isotope that is often used in fission reactions?
Answers: 1
You know the right answer?
The amino acid glycine is often used as the main ingredient of a buffer in biochemical experiments....
Questions

Spanish, 27.07.2019 12:00

Biology, 27.07.2019 12:00

Geography, 27.07.2019 12:00

History, 27.07.2019 12:00

History, 27.07.2019 12:00

Business, 27.07.2019 12:00

History, 27.07.2019 12:00

History, 27.07.2019 12:00



Biology, 27.07.2019 12:00

History, 27.07.2019 12:00


Biology, 27.07.2019 12:00

Chemistry, 27.07.2019 12:00

Physics, 27.07.2019 12:00


English, 27.07.2019 12:00


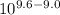
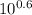
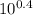
![pH = pKa + log [\dfrac{NH_2}{NH_3^+}]](/tpl/images/0687/6688/3d8e8.png)
![pH = pKa + log [\dfrac{0.01}{0.99}]](/tpl/images/0687/6688/7b228.png)


