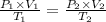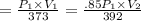Chemistry, 17.06.2020 19:57 deshawnnash53
For many purposes we can treat methane (CH) as an ideal gas at temperatures above its boiling point of - 161. °C. Suppose the temperature of a sample of methane gas is raised from 100.0 °C to 119.0 °C, and at the same time the pressure is decreased by 15.0%. increase Does the volume of the sample increase, decrease, or stay the same? decrease x 6 ? stays the same If you said the volume increases or decreases, calculate the percentage change in the volume. Round your answer to the nearest percent. 1%

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Complete the sentence. the lower the hydrogen ion concentration, the the ph. higher lower closer to 7 closer to 0
Answers: 2

Chemistry, 22.06.2019 07:40
22. a flask containing 450 ml of 0.50 m h2so4 was accidentally knocked to the floor. how many grams of nahco, do you need to put on the spill to neutralize the acid according to the following equation: h2so4(aq)+2 nahcos(aq) na,so(aq) +2 h20()+2 co2(g) d) 38 g a) 2.3 g b) 9.5 g c) 19 g
Answers: 1

Chemistry, 22.06.2019 16:50
Which element is least likely to undergo a chemical reaction
Answers: 3

Chemistry, 22.06.2019 22:30
What relationship exists between an enzyme and a catalyst?
Answers: 1
You know the right answer?
For many purposes we can treat methane (CH) as an ideal gas at temperatures above its boiling point...
Questions

Mathematics, 16.02.2021 22:30


Mathematics, 16.02.2021 22:30

Mathematics, 16.02.2021 22:30

Mathematics, 16.02.2021 22:30


Mathematics, 16.02.2021 22:30


Biology, 16.02.2021 22:30

Mathematics, 16.02.2021 22:30

Physics, 16.02.2021 22:30

Mathematics, 16.02.2021 22:30

Biology, 16.02.2021 22:30



Physics, 16.02.2021 22:30

Mathematics, 16.02.2021 22:30

Chemistry, 16.02.2021 22:30


Mathematics, 16.02.2021 22:30