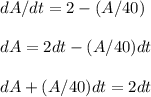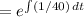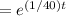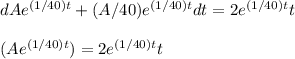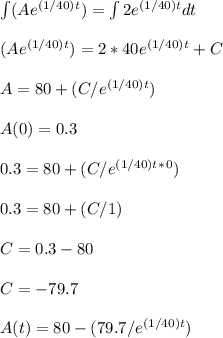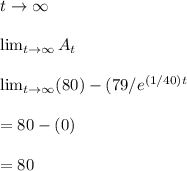Chemistry, 18.06.2020 02:57 melinalange48
A tank contains 200 gallons of water in which 300 grams of salt is dissolved. A brine solution containing 0.4 kilograms of salt per gallon of water is pumped into the tank at the rate of 5 gallons per minute, and the well-stirred mixture is pumped out at the same rate. Let LaTeX: A\left(t\right)A(t)[Math Processing Error] represent the amount of salt (measured in kilograms) in the tank at time LaTeX: t[Math Processing Error].

Answers: 3


Another question on Chemistry

Chemistry, 23.06.2019 00:50
50 points! need answer asap. what type of organic compound contains the following functional group? (2 points)
Answers: 3

Chemistry, 23.06.2019 02:00
Calculate the molarity of each aqueous solution: a. 78.0 ml of 0.240 m naoh diluted to 0.250 l with water b. 38.5 ml of 1.2 m hno3 diluted to 0.130 l with water
Answers: 1

Chemistry, 23.06.2019 05:30
Astudent made the lewis dot diagram of a compound as shown. mg is written with two dots shown on its top. an o is written on each side of mg. each o has six dots around it. an arrow is shown from one dot on mg toward the vacant space around the o on the right. another arrow is shown from the other dot on mg toward the vacant space around the o on the left. the title of the art is students lewis dot model. what is the error in the lewis dot diagram? an o atom should transfer all its six electrons to mg because the formula is mgo. both electrons of mg should be transferred to one o atom because the formula is mgo. the electrons should be transferred from each o atom to mg because mg has fewer electrons. the number of dots around mg should be four because it has to transfer two electrons to each o.
Answers: 2

Chemistry, 23.06.2019 07:00
Under what conditions will a gas be most likely to exhibit the ideal gas properties predicted by the ideal gas law? 1)high pressures and high temperature, because particles are forced closer together with higher kinetic energy, so intermolecular forces of attraction are weaker 2)high pressure and low temperature, because particles are forced closer together and moving slower, so the volume of the particles is less significant 3) low pressure and high temperature, because particles are spread farther apart and moving faster, so the intermolecular forces of attraction are weaker 4)low pressure and low temperature, because particles are spread farther apart with lower kinetic energy, so the volume of the particles is less significant
Answers: 2
You know the right answer?
A tank contains 200 gallons of water in which 300 grams of salt is dissolved. A brine solution conta...
Questions




Mathematics, 12.11.2020 18:10


Mathematics, 12.11.2020 18:10




Mathematics, 12.11.2020 18:10


Computers and Technology, 12.11.2020 18:10


Mathematics, 12.11.2020 18:10





Mathematics, 12.11.2020 18:10