
Chemistry, 17.06.2020 15:57 TH3L0N3W0LF
Consider the substances below.
1 mol of beryllium
• 1 mol of salt
1 mol of water
• 1 mol of hydrogen
Which statement is true about these substances?
They have exactly the same mass.
O They have different numbers of particles.
They have the same number of atoms.
© They have different masses.
Mark this and return
Save and Exit
Next

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 21:30
Liquid ammonia is produced at high temperatures and under great pressure in a tank by passing a mixture of nitrogen gas and hydrogen gas over an iron catalyst. the reaction is represented by the following equation. n2(g) + 3h2(g) → 2nh3(g) changing all but one experimental condition will affect the amount of ammonia produced. that condition is a) increasing the concentration of both reactants b) changing the temperature within the tank c) decreasing the pressure within the tank. d) increasing only the amount of nitrogen present.
Answers: 1

Chemistry, 22.06.2019 22:00
8) warming your hands by a fire is an example if which heat transfer? a. conduction b. convection c. radiation d. none of these
Answers: 1

Chemistry, 22.06.2019 23:00
What is the average rate of the reaction between 10 and 20 s?
Answers: 1

Chemistry, 23.06.2019 21:30
Explain why the epa is not present as defined by weston wilson
Answers: 1
You know the right answer?
Consider the substances below.
1 mol of beryllium
• 1 mol of salt
1 mol of water
...
• 1 mol of salt
1 mol of water
...
Questions


Mathematics, 07.03.2021 22:20

Computers and Technology, 07.03.2021 22:20

Mathematics, 07.03.2021 22:20





Chemistry, 07.03.2021 22:20



Biology, 07.03.2021 22:20

Biology, 07.03.2021 22:20

Health, 07.03.2021 22:20

Mathematics, 07.03.2021 22:20

Mathematics, 07.03.2021 22:20

Health, 07.03.2021 22:20


Mathematics, 07.03.2021 22:20

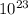 .
.

