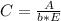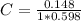Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:50
7. what temperature is need to just dissolve 50 g of nh4cl in 75 g of water? '
Answers: 1

Chemistry, 22.06.2019 16:00
Which factor is likely to impact the possible number of compounds ?
Answers: 1

Chemistry, 23.06.2019 16:10
Which quote from the passage best illustrates della’s optimism? “…and at length she was able to look up with dim eyes and a smile and say: "my hair grows so fast, jim! ” “…her heart had simply craved and yearned over them without the least hope of possession.” “and now, they were hers, but the tresses that should have adorned the coveted adornments were gone.” “beautiful combs, pure tortoise shell, with jewelled rims--just the shade to wear in the beautiful vanished hair.”d, ardent, most likely means? gloomy calm passionate innocent
Answers: 2

Chemistry, 23.06.2019 22:10
Most substances become less dense as the temperature increases. which substance is an exception to this rule? ice at 0°c steel at 40°c oxygen at 20°c aluminum at 20°c
Answers: 3
You know the right answer?
After creating a Beer's Law plot using standard solutions of Q, you determined the slope of Beer's L...
Questions


Mathematics, 23.03.2021 18:30

Mathematics, 23.03.2021 18:30

Mathematics, 23.03.2021 18:30

Mathematics, 23.03.2021 18:30


Mathematics, 23.03.2021 18:30

Social Studies, 23.03.2021 18:30

Mathematics, 23.03.2021 18:30

Health, 23.03.2021 18:30


Mathematics, 23.03.2021 18:30

Mathematics, 23.03.2021 18:30


History, 23.03.2021 18:30

Mathematics, 23.03.2021 18:30

Physics, 23.03.2021 18:30


Geography, 23.03.2021 18:30

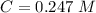
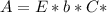 . On this equation we will have:
. On this equation we will have: