
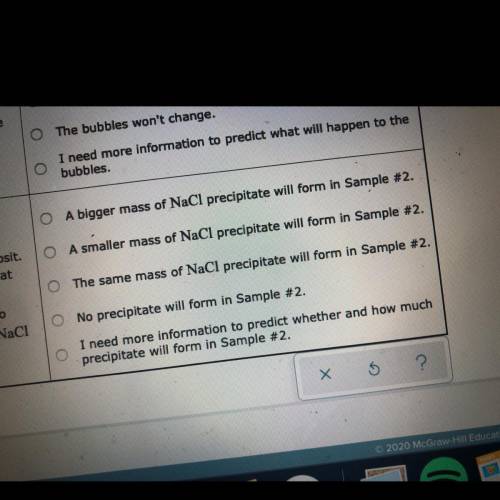
Two 250 mL samples of water are drawn from a deep
well bored into a large underground salt (NaCl) deposit. Sample #1 is from the top of the well, and is initially at 42 °C. Sample #2 is from a depth of 150 m, and is
initially at 8 °C. Both samples are allowed to come to room temperature (20 °C) and 1 atm pressure. An NaCl precipitate is seen to form in Sample #1.
The rest of the question is in the picture!

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 11:00
As air becomes more dense, (select all that apply) o. air weighs less o. gas molecules are closer together o. air is colder o. air weighs more o. gas molecules are further apart o. air is hotter
Answers: 3


Chemistry, 22.06.2019 17:40
Which statement about hf is true? it is zero for any compound in its standard state. it is positive when the bonds of the product store more energy than those of the reactants. it is negative when a compound forms from elements in their standard states. it is zero for any element that is in the liquid state.
Answers: 1
You know the right answer?
Two 250 mL samples of water are drawn from a deep
well bored into a large underground salt (NaCl) d...
Questions

English, 08.07.2019 05:30


Mathematics, 08.07.2019 05:30









English, 08.07.2019 05:30

English, 08.07.2019 05:30




Mathematics, 08.07.2019 05:40


History, 08.07.2019 05:40

Mathematics, 08.07.2019 05:40



