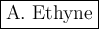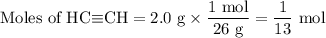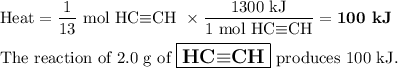Chemistry, 18.06.2020 13:57 cmflores3245
Q1.The table below shows data for the four hydrocarbons ethyne, propyne, propene and propane. AHc is the
standard enthalpy of combustion of these hydrocarbons.
Compound
Name
M
-AHC
/kJ mol
HCECH
ethyne
26
1300
HC-CCH,
propyne
40
1940
H. C=CHCH,
propene
42
2060
CH, CH, CH
propane
44
2220
The complete combustion of 2.0 g of one of the above hydrocarbons releases exactly 100 kJ of
heat energy
This hydrocarbon is
A
ethyne
B
propyne
C
propene
D
propane
(Total 1 mark)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
In order to calculate the amount of heat transferred you must know the __ and specific heat of the material, as well as the change in temperature. a. volume b. density c. mass d. enthalpy
Answers: 1

Chemistry, 21.06.2019 23:00
Why are the trends and exceptions to the trends in ionization energy observed?
Answers: 1

Chemistry, 22.06.2019 04:10
Answer from each drop-down menu. e characteristics of a borane molecule (bh). the lewis structure and table of electronegativities are given olecular shape is and the molecule is reset next erved. search e a
Answers: 2

Chemistry, 22.06.2019 05:00
Frictional forces acting on an object are often converted into energy, which causes the temperature of the object to rise slightly.
Answers: 2
You know the right answer?
Q1.The table below shows data for the four hydrocarbons ethyne, propyne, propene and propane. AHc is...
Questions


English, 16.04.2020 16:21





Mathematics, 16.04.2020 16:21

Geography, 16.04.2020 16:21

Health, 16.04.2020 16:21


Mathematics, 16.04.2020 16:21





Mathematics, 16.04.2020 16:21



Mathematics, 16.04.2020 16:21