
In the first step of glycolysis, the given two reactions are coupled. reaction 1:reaction 2:glucose+Pi⟶glucose-6-phosphate+H2 OATP+H2O⟶ADP+PiΔG=+13.8 kJ/molΔG=−30.5 kJ/mol reaction 1:glucose+Pi⟶glucose-6-phosphate+H2 OΔG=+13.8 kJ/molreaction 2:ATP+H2O⟶ADP+PiΔG=−30.5 kJ/mol Answer the four questions about the first step of glycolysis. Is reaction 2 spontaneous or nonspontaneous?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 14:40
What type of solution is formed if 10 g of kclo3 are dissolved in 100g of water at 30
Answers: 2

Chemistry, 22.06.2019 05:30
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 10:30
Which describes fat? a: a carbohydrate that produces energy b: a nucleic acid that directs cell function c: a lipid that stores energy d: a protein that speeds up a chemical reaction
Answers: 1

Chemistry, 22.06.2019 12:20
The yearly amounts of carbon emissions from cars in belgium are normally distributed with a mean of 13.9 gigagrams per year and a standard deviation of 5.8 gigagrams per year. find the probability that the amount of carbon emissions from cars in belgium for a randomly selected year are between 11.5 gigagrams and 14.0 gigagrams per year. a. 0.340 b. 0.660 c. 0.167 d. 0.397
Answers: 2
You know the right answer?
In the first step of glycolysis, the given two reactions are coupled. reaction 1:reaction 2:glucose+...
Questions

Mathematics, 13.12.2020 08:20

English, 13.12.2020 08:20


History, 13.12.2020 08:20

Chemistry, 13.12.2020 08:20


Mathematics, 13.12.2020 08:20

Spanish, 13.12.2020 08:20

Mathematics, 13.12.2020 08:20



Physics, 13.12.2020 08:20

Mathematics, 13.12.2020 08:20

Chemistry, 13.12.2020 08:20

Mathematics, 13.12.2020 08:20

Mathematics, 13.12.2020 08:20



English, 13.12.2020 08:20

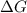 = +ve, reaction is non spontaneous
= +ve, reaction is non spontaneous
 glucose-6-phosphate + H₂O, ΔG = +13.8 kJ/mol
glucose-6-phosphate + H₂O, ΔG = +13.8 kJ/mol

