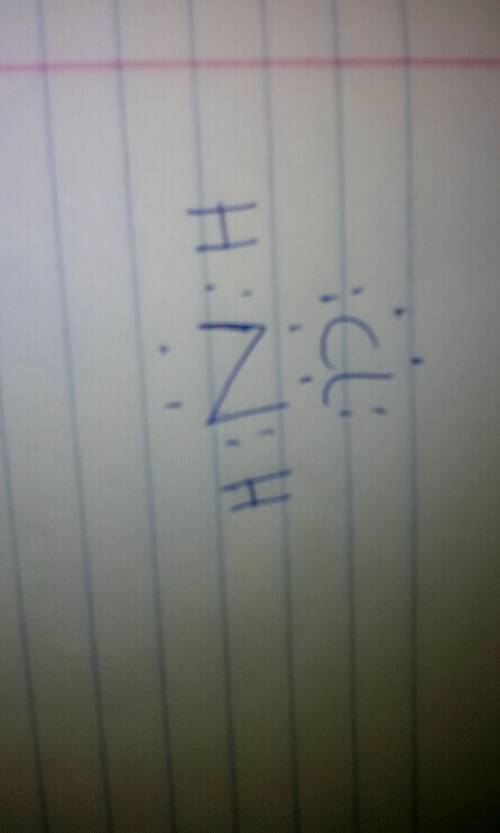Chemistry, 18.06.2020 22:57 Ameilasmickle15
Drag the tiles to the correct locations. Each tile can be used more than once, but not all tiles will be used. Some locations will remain empty.
Chloramine has the chemical formula NH, CI. Nitrogen has five valence electrons, each hydrogen has one valence electron, and chlorine has
seven valence electrons. Complete the Lewis structure for this covalent compound.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 14:30
How do temperature and salinity affect deepwater currents? as temperatures and salinity levels of water increase, the water rises to the surface where it creates currents as it moves to colder regions. they create changes in wind direction, moving denser water in the same direction as the wind and causing the deepwater circulation patterns found in the ocean. they equalize the forces on undersea currents caused by the coriolis effect as they replace more dense water with less dense water. they create density differences that cause dense deepwater currents to flow toward the equator where they displace less dense, warmer water above them.
Answers: 2

Chemistry, 22.06.2019 16:50
Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of water to produce a solution that freezes at -14.5 c? the freezing point for pure water is 0.0 c and k_f is equal to 1.86 c/m
Answers: 3

Chemistry, 23.06.2019 09:20
Due tomorrow which would have a lower ph, a 0.1 m solution of a strong base or a weak base? why? which would have a higher ph, a 0.1 m solution of a strong base or a weak base? why?
Answers: 3
You know the right answer?
Drag the tiles to the correct locations. Each tile can be used more than once, but not all tiles wil...
Questions

History, 19.09.2019 00:30


Mathematics, 19.09.2019 00:30




Mathematics, 19.09.2019 00:40




Social Studies, 19.09.2019 00:40


Mathematics, 19.09.2019 00:40


Biology, 19.09.2019 00:40

Biology, 19.09.2019 00:40

History, 19.09.2019 00:40

Mathematics, 19.09.2019 00:40

Physics, 19.09.2019 00:40

Mathematics, 19.09.2019 00:40