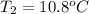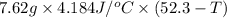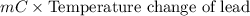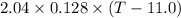Chemistry, 19.06.2020 00:57 straightbarz5916
A 2.04 g lead weight, initially at 10.8 oC, is submerged in 7.62 g of water at 52.3 oC in an insulated container. clear = 0.128 J/g oF; water = 4.18 J/goC. What is the final temperature of both the weight and the water at thermal equilibrium

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 15:30
Which of the following are correct values for the ideal gas laws constant r
Answers: 1

Chemistry, 22.06.2019 18:10
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1

Chemistry, 22.06.2019 20:30
Draw a line graph showing the relationship between temperature in kelvin as a function of kinetic energy.
Answers: 3

Chemistry, 23.06.2019 02:00
Calculate the molarity of each aqueous solution: a. 78.0 ml of 0.240 m naoh diluted to 0.250 l with water b. 38.5 ml of 1.2 m hno3 diluted to 0.130 l with water
Answers: 1
You know the right answer?
A 2.04 g lead weight, initially at 10.8 oC, is submerged in 7.62 g of water at 52.3 oC in an insulat...
Questions

Mathematics, 25.09.2019 21:20

English, 25.09.2019 21:20

Chemistry, 25.09.2019 21:20

Biology, 25.09.2019 21:20






English, 25.09.2019 21:20


Business, 25.09.2019 21:20

Mathematics, 25.09.2019 21:20



Physics, 25.09.2019 21:20

Arts, 25.09.2019 21:30

Health, 25.09.2019 21:30

Mathematics, 25.09.2019 21:30

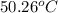 .
.