Chemistry, 19.06.2020 00:57 josephvcarter
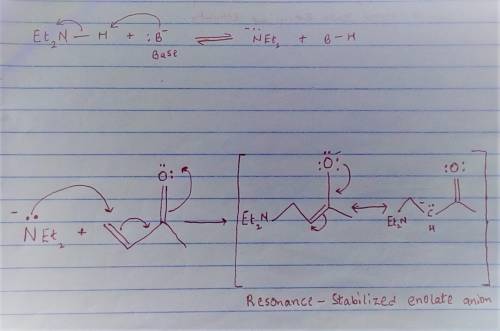
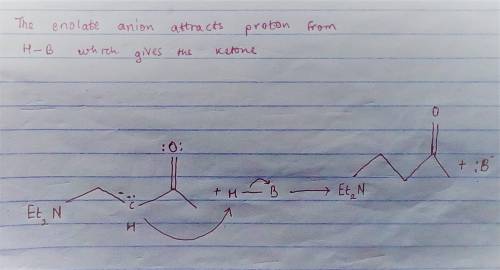
Carbon-carbon double bonds are electron-rich regions and are attacked by electrophiles (for example, ); they are not attacked by nucleophiles (for example, diethylamine, ). + reaction arrow with electrophilic addition written above + no reaction However, when the carbon-carbon double bond has a carbonyl group adjacent to it, the double bond reacts readily with nucleophiles by nucleophilic addition. + reaction arrow with nucleophilic addition written above For the following reaction, draw the structure of the resonance contributor that is attacked by diethylamine. + Include all valence lone pairs in your answer.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:50
In which situation can a mixture always be called a solution
Answers: 3

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 13:00
Is 9 correct? and can someone me with 10? it’s due tomorrow, you
Answers: 1

Chemistry, 22.06.2019 23:30
The sum of the oxidation numbers in a neutral compound is always
Answers: 2
You know the right answer?
Carbon-carbon double bonds are electron-rich regions and are attacked by electrophiles (for example,...
Questions



Physics, 22.02.2021 14:10


German, 22.02.2021 14:20


Mathematics, 22.02.2021 14:20


History, 22.02.2021 14:20

Business, 22.02.2021 14:20

Mathematics, 22.02.2021 14:20

Mathematics, 22.02.2021 14:20

English, 22.02.2021 14:20

Mathematics, 22.02.2021 14:20




Mathematics, 22.02.2021 14:20


Chemistry, 22.02.2021 14:20

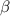 - carbon of the conjugate thereby resulting into a resonance stabilized enolate anion.
- carbon of the conjugate thereby resulting into a resonance stabilized enolate anion.