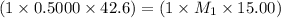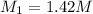Chemistry, 18.06.2020 15:57 umezinwachukwuebuka1
Ammonia (NH3) is a weak base that reacts with a strong acid to form the ammonium ion, NH4 . In the titration of 15.00 mL of an ammonia cleaner with 0.5000 M HCl, 42.6 mL of the titrant was required to reach the endpoint. What is the concentration of the NH3 in solution

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
You have 125g of a certain seasoning and are told that it contains 76.0 g of salt what is the percentage of salt by mass in this seasoning
Answers: 1


Chemistry, 22.06.2019 16:00
Inside a flashbulb, oxygen surrounds a thin coil of magnesium. when the flashbulb is set off, a chemical reaction takes place in which magnesium combines with oxygen to form magnesium oxide. which of the chemical equations matches the reaction above? a. mg + o2 mgo2 + energy b. 2mg + o mg2o + energy c. 2mg + o2 2mgo + energy d. mg + o mgo + energy
Answers: 1

Chemistry, 22.06.2019 22:10
Which aqueous solution of ki freezes at the lowest temperature? 1) 1 mol of ki in 500. g of water 2) 2 mol of ki in 500. g of water 3) 1 mol of ki in 1000. g of water 4) 2 mol of ki in 1000. g of water
Answers: 3
You know the right answer?
Ammonia (NH3) is a weak base that reacts with a strong acid to form the ammonium ion, NH4 . In the t...
Questions


Computers and Technology, 06.11.2020 16:50



Arts, 06.11.2020 16:50



Mathematics, 06.11.2020 16:50

Arts, 06.11.2020 16:50

Mathematics, 06.11.2020 16:50

Mathematics, 06.11.2020 16:50




Biology, 06.11.2020 16:50

Arts, 06.11.2020 16:50


Mathematics, 06.11.2020 16:50


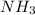 in solution is 1.42 M
in solution is 1.42 M
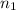 = basicity of
= basicity of 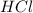 = 1
= 1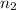 = acidity of
= acidity of 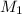 = concentration of
= concentration of 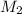 = concentration of
= concentration of 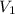 = volume of
= volume of 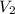 = volume of
= volume of