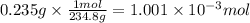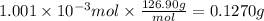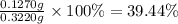Chemistry, 19.06.2020 03:57 smartboy2296
A sample of 0.3220 g of an ionic compound containing the iodide ion (I-) is dissolved in water and treated with an excess of AgHCO3. If the mass of the AgI precipitate that forms is 0.235 g, what is the percent by mass of I in the original compound? The molar mass of AgI is 234.8 g.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:10
Think about how you can use le chatelier’s principle to find possible solutions to the design problem. describe at least two ways to increase the yield (amount) of ammonia based on this principle.
Answers: 2

Chemistry, 22.06.2019 10:30
Which characteristics can be used to differentiate star systems? check all that apply.
Answers: 2


Chemistry, 22.06.2019 23:30
If it is an isoelectronic series select true, if not select false. o2-, s2-, se2-, te2- na+, k+, rb+, cs+ n3-, p3-, as3-, sb3- ag, cd+, sn3+, sb4+ f-, cl-, br-, i- f-, ne, na+, mg2+ s2-, s, s6+
Answers: 1
You know the right answer?
A sample of 0.3220 g of an ionic compound containing the iodide ion (I-) is dissolved in water and t...
Questions

Social Studies, 06.05.2020 20:39


Mathematics, 06.05.2020 20:39


Mathematics, 06.05.2020 20:39

History, 06.05.2020 20:39

History, 06.05.2020 20:39


Mathematics, 06.05.2020 20:39

Health, 06.05.2020 20:39


Chemistry, 06.05.2020 20:39

Computers and Technology, 06.05.2020 20:39


Mathematics, 06.05.2020 20:39


Mathematics, 06.05.2020 20:39

Mathematics, 06.05.2020 20:39

Mathematics, 06.05.2020 20:39