Step 7: Measuring Masses of the Reactants
(Reaction 2)
Mass of HCI:
g
Mass of sol...

Chemistry, 19.06.2020 06:57 coryintheswamp
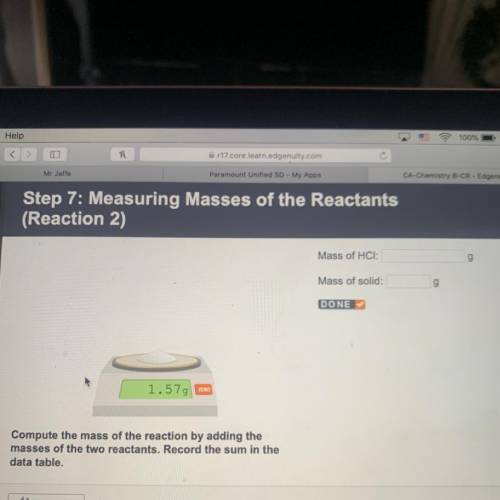
Step 7: Measuring Masses of the Reactants
(Reaction 2)
Mass of HCI:
g
Mass of solid:
g
DONE
1.579 zxo
Compute the mass of the reaction by adding the
masses of the two reactants. Record the sum in the
data table.
(need asap)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
This question is about electrolysis. metal spoons can be coated with silver. this is called electroplating. suggest one reason why spoons are electroplated?
Answers: 1

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 1

Chemistry, 22.06.2019 17:30
Upon decomposition, one sample of magnesium fluoride produced 1.65 kg of magnesium and 2.56 kg of fluorine. a second sample produced 1.32 kg of magnesium. part a how much fluorine (in grams) did the second sample produce?
Answers: 2

You know the right answer?
Questions

Mathematics, 23.08.2019 06:10


History, 23.08.2019 06:10

Social Studies, 23.08.2019 06:10

History, 23.08.2019 06:10



Mathematics, 23.08.2019 06:10




Mathematics, 23.08.2019 06:10

Mathematics, 23.08.2019 06:10

Mathematics, 23.08.2019 06:10


Mathematics, 23.08.2019 06:10

Mathematics, 23.08.2019 06:10

Mathematics, 23.08.2019 06:10

Chemistry, 23.08.2019 06:10



