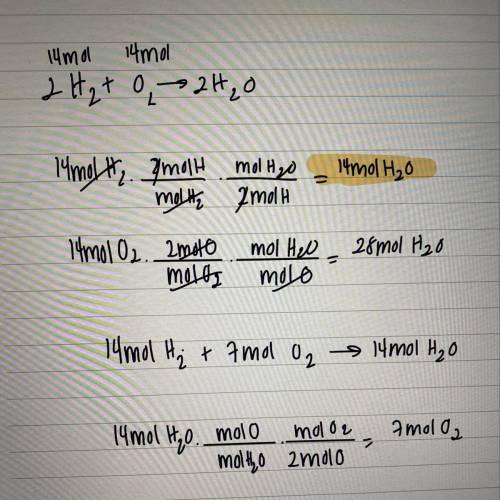Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:30
Aaspirin has a density of 1.40 g/cm^3 what is the volume in cubic centimeters of a tablet weighing 320 mg?
Answers: 3

Chemistry, 22.06.2019 08:00
Will give ! what are the advantages and disadvantages of nuclear power? check all that apply. one advantage of nuclear energy is that it does not produce carbon dioxide emissions. storage of nuclear waste is a short-term problem associated with nuclear energy. the problem with uranium mining is that a large quantity of uranium must be extracted to meet energy needs because the energy release from uranium fission is so low. safe operation of a nuclear power plant can be jeopardized by a human mistake.
Answers: 1

Chemistry, 22.06.2019 09:40
Apiece of copper has a temperature of 75.6 0c. when the metal is placed in 100.0 grams of water at 19.1 0c, the temperature rises by 5.5 0c. what is the mass of the metal?
Answers: 1

Chemistry, 22.06.2019 23:10
Using the periodic table, complete the following. element: hydrogen symbol: h₂ molecular weight: g mass of one mole: g/mol
Answers: 3
You know the right answer?
If 14 moles of oxygen react with 14 moles of hydrogen to produce water, what is the
limiting reacta...
Questions



Business, 18.06.2021 20:10


Mathematics, 18.06.2021 20:10

Mathematics, 18.06.2021 20:10

Mathematics, 18.06.2021 20:10

Mathematics, 18.06.2021 20:10

Physics, 18.06.2021 20:10

Mathematics, 18.06.2021 20:10

English, 18.06.2021 20:10

Social Studies, 18.06.2021 20:10


Mathematics, 18.06.2021 20:10

Mathematics, 18.06.2021 20:10

Mathematics, 18.06.2021 20:10

English, 18.06.2021 20:10

Mathematics, 18.06.2021 20:10

Mathematics, 18.06.2021 20:10