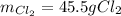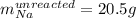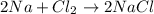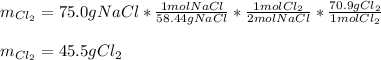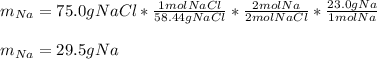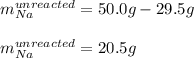A reaction vessel contains 50.0 grams of sodium metal and chlorine gas. How many grams of chlorine gas are needed to produce 75.0 grams of sodium chloride? If this amount of chlorine gas is added to the vessel, how much sodium metal would remain after the reaction goes to completion?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 3

Chemistry, 22.06.2019 22:30
[ou.03jthe pictures below show the wavelengths and intensities of electromagnetic radiations emitted by three stars, star 1, star 2, and star 3. intensity intensity- intensity- 1000 3500 6000 8500 11000 wavelength (a) star 1 1000 3500 6000 8500 11000 1000 3500 6000 8500 11000 wavelength (a) wavelength (a) star 2 star 3 which of these statements is correct about the color of the three stars? star 2 is white in color o star 2 is yellow in color star 1 and star 3 are yellow in color star 1 and star 3 are white in color
Answers: 1

Chemistry, 23.06.2019 02:30
Ascientist wants to know how individual lions within a pride interact with each other in their own environment. to do this, the scientist sedates and tags all of the lions within a pride. then, he places several remotely-controlled video cameras near the lions' den and performs an observational field study. he collects continuous video footage over the span of one year, analyzes the video, and then forms conclusions based on his observations.
Answers: 2
You know the right answer?
A reaction vessel contains 50.0 grams of sodium metal and chlorine gas. How many grams of chlorine g...
Questions

Chemistry, 18.03.2021 01:40




Mathematics, 18.03.2021 01:40

Mathematics, 18.03.2021 01:40

History, 18.03.2021 01:40




Mathematics, 18.03.2021 01:40





History, 18.03.2021 01:40


Arts, 18.03.2021 01:40


Chemistry, 18.03.2021 01:40