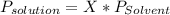Chemistry, 20.06.2020 05:57 kodiebclay
The vapor pressure of diethyl ether (ether) is 463.6 mm Hg at 25 °C. How many grams of aspirin , C9H8O4, a nonvolatile, nonelectrolyte (MW = 180.1 g/mol), must be added to 219.0 grams of diethyl ether to reduce the vapor pressure to 458.5 mm Hg ? diethyl ether = CH3CH2OCH2CH3 = 74.12 g/mol.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which of these properties, used alone, would be least useful in identifying most minerals? a. color b. luster c. streak d. density
Answers: 2

Chemistry, 22.06.2019 15:10
The ozone molecule o3 has a permanent dipole moment of 1.8×10−30 cm. although the molecule is very slightly bent-which is why it has a dipole moment-it can be modeled as a uniform rod of length 2.5×10−10 m with the dipole moment perpendicular to the axis of the rod. suppose an ozone molecule is in a 8000 n/c uniform electric field. in equilibrium, the dipole moment is aligned with the electric field. but if the molecule is rotated by a small angle and released, it will oscillate back and forth in simple harmonic motion.what is the frequency f of oscillation?
Answers: 2

Chemistry, 22.06.2019 18:00
How does climate change cause the ocean's thermohaline current to slow down?
Answers: 3

You know the right answer?
The vapor pressure of diethyl ether (ether) is 463.6 mm Hg at 25 °C. How many grams of aspirin , C9H...
Questions


Mathematics, 03.09.2020 19:01

Medicine, 03.09.2020 19:01

Mathematics, 03.09.2020 19:01

Mathematics, 03.09.2020 19:01

Geography, 03.09.2020 19:01


Mathematics, 03.09.2020 19:01


Social Studies, 03.09.2020 19:01

History, 03.09.2020 19:01

Mathematics, 03.09.2020 19:01

Chemistry, 03.09.2020 19:01



Spanish, 03.09.2020 19:01

History, 03.09.2020 19:01