Using the rules of significant figures, calculate the following: 1.2315+
0.116 + 15.10 is.
16...

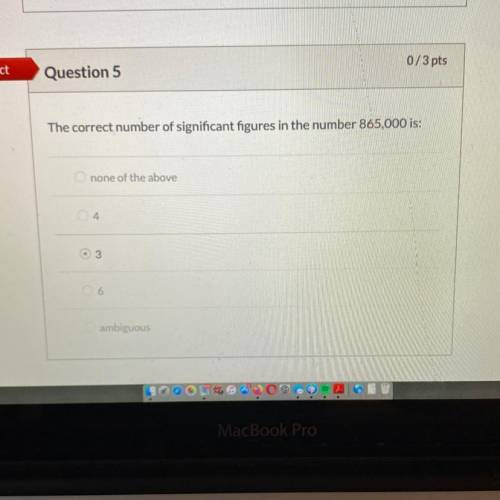
Chemistry, 19.06.2020 07:57 lightbright828
Using the rules of significant figures, calculate the following: 1.2315+
0.116 + 15.10 is.
16.45
• 16.4
16
16.4475
0/3 nts

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:10
According to the diagram; a) identify the anode of the cell and write the half-reaction that occurs there b) write the overall equation for the reaction that occurs as the cell operates c) calculate the value of the standard cell potential ,e cell. d)write the shorthand notation of the cell above e)indicate the flow of the electrons on the diagram
Answers: 3

Chemistry, 22.06.2019 00:30
Jessica is traveling from miami, florida, to chicago, illinois. using the map, tell one way the land will change during the second half of her trip.
Answers: 1

Chemistry, 22.06.2019 11:00
Imagine that twenty i.u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2

Chemistry, 22.06.2019 12:30
Suppose you wanted to make 100 grams of water. what is the molar mass of water (h2o)?
Answers: 2
You know the right answer?
Questions

Mathematics, 15.04.2020 22:08










Mathematics, 15.04.2020 22:08

Computers and Technology, 15.04.2020 22:08

History, 15.04.2020 22:08





Spanish, 15.04.2020 22:08

Mathematics, 15.04.2020 22:08



