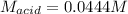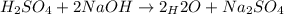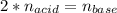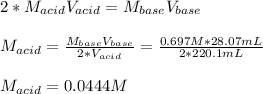Suppose you are titrating a sulfuric acid solution of unknown concentration with a sodium hydroxide solution according to the equation H 2 S O 4 + 2 N a O H ⟶ 2 H 2 O + N a 2 S O 4 If you require 28.07 mL of 0.697 M NaOH solution to titrate 220.1 mL of H 2 SO 4 solution, what is the concentration of the H 2 SO 4 solution? Type

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 10:00
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1


Chemistry, 22.06.2019 16:00
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1
You know the right answer?
Suppose you are titrating a sulfuric acid solution of unknown concentration with a sodium hydroxide...
Questions





Mathematics, 15.12.2020 23:10

Mathematics, 15.12.2020 23:10


Mathematics, 15.12.2020 23:10

Mathematics, 15.12.2020 23:10




Mathematics, 15.12.2020 23:10

Mathematics, 15.12.2020 23:10

Mathematics, 15.12.2020 23:10

Computers and Technology, 15.12.2020 23:10

English, 15.12.2020 23:10