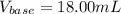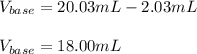Chemistry, 20.06.2020 17:57 seaotter7140
uppose you are titrating an acid of unknown concentration with a standardized base. At the beginning of the titration, you read the base titrant volume as 2.03 mL. After running the titration and reaching the endpoint, you read the base titrant volume as 20.03 mL. What volume of base was required for the titration

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:00
An electron moved from shell n = 2 to shell n = 1. what most likely happened during the transition? a fraction of a photon was added. a photon of energy was absorbed. a fraction of a photon was removed. a photon of energy was released.
Answers: 1

Chemistry, 23.06.2019 00:00
What is the pressure of 0.500 moles of carbon dioxide gas in a 2.5 l tank and at a temperature of 301 k? (r=0.0821 l·atm/mol·k) 3.08 atm 1.2 atm 0.23 atm 4.01 atm 4.94 atm
Answers: 1

Chemistry, 23.06.2019 02:00
Which best describes the present-day universe? opaque, expanding very slowly, stars produce heavy elements transparent, expanding at an accelerated rate, stars produce heavy elements opaque, expanding at an accelerated rate, stars produce only hydrogen and helium transparent, expanding very slowly, stars produce only hydrogen and helium
Answers: 1

Chemistry, 23.06.2019 03:50
Show how to convert the temperature 84.7° c to kelvin. include all steps and label the final answer
Answers: 1
You know the right answer?
uppose you are titrating an acid of unknown concentration with a standardized base. At the beginning...
Questions

Computers and Technology, 01.12.2020 01:00



Mathematics, 01.12.2020 01:00




History, 01.12.2020 01:00


Mathematics, 01.12.2020 01:00



English, 01.12.2020 01:00


Mathematics, 01.12.2020 01:00



Health, 01.12.2020 01:00

English, 01.12.2020 01:00