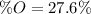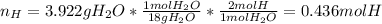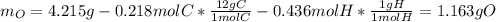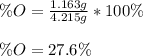Chemistry, 20.06.2020 17:57 collin0123
A 4.215 g sample of a compound containing only carbon, hydrogen, and oxygen is burned in an excess of oxygen gas, producing 9.582 g CO2 and 3.922 g H2O. What percent by mass of oxygen is contained in the original sample?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:40
Which of the following pressures is equal to 760 mm hg? 2.0 atm 101.3 pa 101,300 kpa 101,300 pa
Answers: 2

Chemistry, 22.06.2019 02:20
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 06:00
What type of electromagnetic radiation has a shorter wavelength than blue light
Answers: 2

Chemistry, 22.06.2019 07:30
In the particles are arranged in a regular, repeating pattern. a)a crystalline liquid b)a crystalline solid c)all gases d)all solids
Answers: 2
You know the right answer?
A 4.215 g sample of a compound containing only carbon, hydrogen, and oxygen is burned in an excess o...
Questions


Mathematics, 21.02.2020 06:24

Mathematics, 21.02.2020 06:24


Mathematics, 21.02.2020 06:24

Mathematics, 21.02.2020 06:24

Geography, 21.02.2020 06:24

Mathematics, 21.02.2020 06:24

Mathematics, 21.02.2020 06:24

Advanced Placement (AP), 21.02.2020 06:24


Mathematics, 21.02.2020 06:24

Mathematics, 21.02.2020 06:24

Health, 21.02.2020 06:24

Chemistry, 21.02.2020 06:24

Mathematics, 21.02.2020 06:24



Social Studies, 21.02.2020 06:24

Mathematics, 21.02.2020 06:24