
Chemistry, 27.09.2019 06:30 niyyyareligion
If 5 moles of p4 reacted with 22 moles cl2 according to the above reaction, determine:
a) how many moles pcl3 are produced
b) how many moles of p4 are left in excess after the reaction (if any)
c) how many moles of cl2 are left in excess after the reaction (if any)

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 12:30
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1

Chemistry, 22.06.2019 23:00
The data below were determined for the reaction shown below. s2o82– + 3i – (aq) → 2so42– + i3– expt. # [s2o82–] (m) [i –] (m) initial rate 1 0.038 0.060 1.4 × 10 – 5 m/s 2 0.076 0.060 2.8 × 10 – 5 m/s 3 0.076 0.030 1.4 × 10 – 5 m/s the rate law for this reaction must be:
Answers: 1

Chemistry, 23.06.2019 04:00
If you are told to get 100 ml of stock solution to use to prepare smaller size sample for an experiment, which piece of glassware would you use?
Answers: 3
You know the right answer?
If 5 moles of p4 reacted with 22 moles cl2 according to the above reaction, determine:
a) how...
a) how...
Questions

English, 23.04.2020 23:58

Mathematics, 23.04.2020 23:58

Biology, 23.04.2020 23:58


Geography, 23.04.2020 23:58

World Languages, 23.04.2020 23:58


English, 23.04.2020 23:58







Mathematics, 23.04.2020 23:58



Mathematics, 23.04.2020 23:58


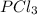 = 2.44 moles
= 2.44 moles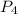 left = 1.34 mole
left = 1.34 mole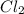 because it is completely consumed in the reaction.
because it is completely consumed in the reaction.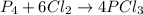
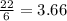 moles of
moles of 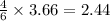 moles of
moles of 


