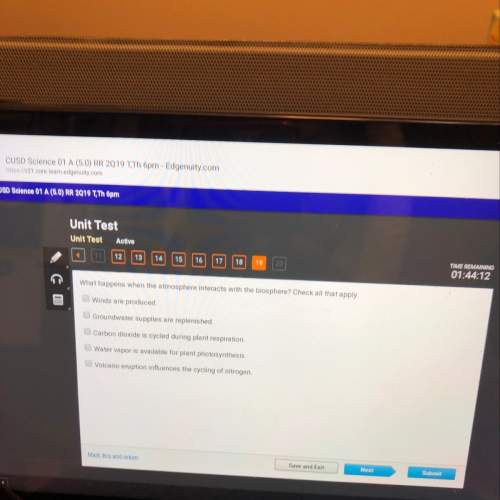
Chemistry, 21.06.2020 01:57 wananikwecurley99
A 10.0 g sample of an unknown liquid is vaporized at 120.0°C and 5.0 atm. The volume of the vapour is found to be 568.0 mL. The liquid is determined to be made up of 84.2% carbon and 15.8% hydrogen. What is the molecular formula for the liquid?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:00
3. if the dartboard below is used to model an atom, which subatomic particles would be located at z?
Answers: 2

Chemistry, 21.06.2019 20:20
Calculate the molarity of the solution. 6.02 x 1022 molecules of hci (molecular weight = 36.5 g/mole) in 2.0 liters of water m
Answers: 1

Chemistry, 22.06.2019 03:10
Agas diffuses 1/7 times faster than hydrogen gas (h2). what is the molar mass of the gas? 100.10 g/mol 98.78 g/mol 86.68 g/mol 79.98 g/mol
Answers: 3

Chemistry, 22.06.2019 10:30
Apiece of metal with a length of 1.42 cm was measured using four different instruments. which of the following measurements is the most accurate?
Answers: 3
You know the right answer?
A 10.0 g sample of an unknown liquid is vaporized at 120.0°C and 5.0 atm. The volume of the vapour i...
Questions

Mathematics, 18.12.2020 02:20



Computers and Technology, 18.12.2020 02:20

Mathematics, 18.12.2020 02:20


Mathematics, 18.12.2020 02:20

Business, 18.12.2020 02:20

Social Studies, 18.12.2020 02:20

Mathematics, 18.12.2020 02:20

English, 18.12.2020 02:20



Physics, 18.12.2020 02:20


Mathematics, 18.12.2020 02:20

Computers and Technology, 18.12.2020 02:20

Mathematics, 18.12.2020 02:20

Computers and Technology, 18.12.2020 02:20

Mathematics, 18.12.2020 02:20