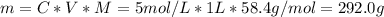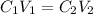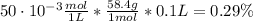Chemistry, 21.06.2020 04:57 trevinojazzy8625
You have a 5 M NaCl stock solution. (NaCl F. W. is 58.4) a) How much NaCl (in g) do you need to make 1 liter of the 5 M stock solution? b) How much stock solution will you need if you want to make 100 ml of 50 mM NaCl? c) What is the % concentration of a 50 mM solution of NaCl?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:00
According to the tide table below what time of day will the highest tide occur? (2 pt) the highest tide will occur at
Answers: 1

Chemistry, 22.06.2019 10:20
Gwhich r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? which r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? −ch2−oh −ch2−o||c−nh2 −ch2−coo− −ch2−ch2−ch2−ch2−n+h3
Answers: 3

Chemistry, 22.06.2019 15:30
The identities of substances are the same before and after which type of change
Answers: 1

Chemistry, 22.06.2019 16:30
How many grams of mgbr2 are needed to produce 75g or metal?
Answers: 1
You know the right answer?
You have a 5 M NaCl stock solution. (NaCl F. W. is 58.4) a) How much NaCl (in g) do you need to make...
Questions

English, 02.04.2021 04:10



History, 02.04.2021 04:10


Mathematics, 02.04.2021 04:20

Chemistry, 02.04.2021 04:20


Mathematics, 02.04.2021 04:20

Mathematics, 02.04.2021 04:20



Mathematics, 02.04.2021 04:20

Mathematics, 02.04.2021 04:20



Mathematics, 02.04.2021 04:20

Mathematics, 02.04.2021 04:20

Law, 02.04.2021 04:20

History, 02.04.2021 04:20