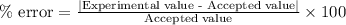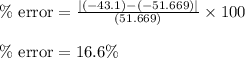Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:00
An observation that requires measurement is called quantitative observable or qualitative
Answers: 1

Chemistry, 22.06.2019 08:30
Which part of earth’s surface receives the most direct rays from the sun? a) equator b) ocean c) poles d) mountains
Answers: 2

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 11:00
Imagine that twenty i.u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2
You know the right answer?
If the theoretical value for AH of the reaction HCl + NH 3 NH 4 Cl is -51.669 kJ/mol, but from your...
Questions

Mathematics, 06.09.2020 03:01

Physics, 06.09.2020 03:01

Mathematics, 06.09.2020 03:01


Mathematics, 06.09.2020 03:01

Social Studies, 06.09.2020 03:01

Health, 06.09.2020 03:01

Mathematics, 06.09.2020 03:01

Chemistry, 06.09.2020 03:01

English, 06.09.2020 03:01

Mathematics, 06.09.2020 03:01

English, 06.09.2020 03:01




Chemistry, 06.09.2020 03:01

Spanish, 06.09.2020 03:01

Mathematics, 06.09.2020 03:01


Biology, 06.09.2020 03:01