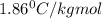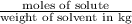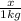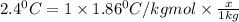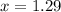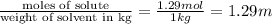Chemistry, 22.06.2020 00:57 Tianylee2328
How many moles of ethylene glycol must be added to 1 kg of water to make a solution with a freezing point of -2.4°C? The freezing point depression constant for water is 1.86°C•kg/mol. What is the molality of the solution?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Agood hypothesis includes which of the following? a: prediction b: data c: uncertainty d: conclusion
Answers: 1

Chemistry, 22.06.2019 04:30
Suppose that during that icy hot lab 65,000 j of energy were transferred to 450 g of water at 20°c what would have have been the final temperature of the water
Answers: 2

Chemistry, 22.06.2019 07:00
Which set of characteristics best describes igneous rock? a) largest type of rock, made of organic matter, hardest type of rock b) least abundant type of rock, made of other rocks, made mostly of minerals c) found on all continents, contains wavy bands of stripes, contains fossils d) most abundant type in earth's crust, made of magma/lava, contains no fossils
Answers: 1

You know the right answer?
How many moles of ethylene glycol must be added to 1 kg of water to make a solution with a freezing...
Questions

Mathematics, 14.05.2021 01:30

Biology, 14.05.2021 01:30

Mathematics, 14.05.2021 01:30

Mathematics, 14.05.2021 01:30




Mathematics, 14.05.2021 01:30





Mathematics, 14.05.2021 01:30



Biology, 14.05.2021 01:30



Mathematics, 14.05.2021 01:30

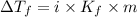
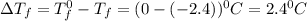 = Depression in freezing point
= Depression in freezing point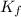 = freezing point constant for water=
= freezing point constant for water=