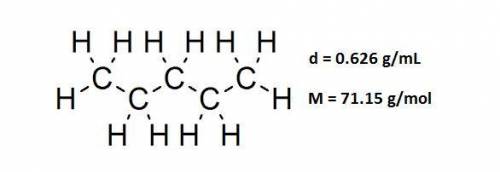Chemistry, 22.06.2020 06:57 MissSusan2253
A beaker contains 41.3 mL of pentane ( C5H12 , density is 0.626 g/mL). Determine how many C atoms this liquid contains.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
It takes 945.kj/mol to break a nitrogen-nitrogen triple bond. calculate the maximum wavelength of light for which a nitrogen-nitrogen triple bond could be broken by absorbing a single photon.
Answers: 3

Chemistry, 22.06.2019 06:00
In an investigation that uses the scientific method, which step immediately follows making a hypothesis? o summarizing the results o asking a question o making observations designing an experiment mark this and retum save and exit next submit
Answers: 2

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 19:00
How many liters of ethylene glycol antifreeze (c2h6o2), with a density of 1.100 g/l, would you add to your car radiator containing 15.0 kg of water if you needed to protect your engine to - 21.5°c? for water, kf = 1.86°c m -1.
Answers: 1
You know the right answer?
A beaker contains 41.3 mL of pentane ( C5H12 , density is 0.626 g/mL). Determine how many C atoms th...
Questions


Mathematics, 03.03.2020 19:03



Social Studies, 03.03.2020 19:03





Mathematics, 03.03.2020 19:03

Computers and Technology, 03.03.2020 19:03

Biology, 03.03.2020 19:03

History, 03.03.2020 19:03




Mathematics, 03.03.2020 19:04


Mathematics, 03.03.2020 19:04

Mathematics, 03.03.2020 19:04