Chemistry, 23.06.2020 17:01 jluckie080117
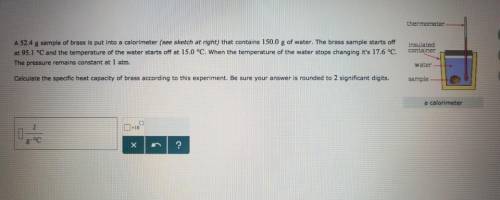
A sample of brass is put into a calorimeter (see sketch at right) that contains of water. The brass sample starts off at and the temperature of the water starts off at . When the temperature of the water stops changing it's . The pressure remains constant at . Calculate the specific heat capacity of brass according to this experiment. Be sure your answer is rounded to significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
If 3.00 g of titanium metal is reacted with 6.00 g of chlorine gas, cl2, to form 7.7 g of titanium (iv) chloride in a combination reaction, what is the percent yield of the product?
Answers: 1


Chemistry, 22.06.2019 05:00
Agas can holds 2.0 gal of gasoline. what is this quantity in cubic centimeters?
Answers: 2

Chemistry, 22.06.2019 09:00
Acrystal that absorvd water from air is (blank)a. aqueousb. homogenousc. hygroscopicd. efflorescent
Answers: 1
You know the right answer?
A sample of brass is put into a calorimeter (see sketch at right) that contains of water. The brass...
Questions




English, 04.01.2022 05:50




Physics, 04.01.2022 05:50

Social Studies, 04.01.2022 05:50

Mathematics, 04.01.2022 05:50




SAT, 04.01.2022 06:00

Mathematics, 04.01.2022 06:00



Mathematics, 04.01.2022 06:00

History, 04.01.2022 06:00

Geography, 04.01.2022 06:00


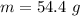


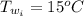
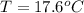

![H_L = m * c_b * [T_i - T]](/tpl/images/0692/3585/a3617.png)
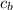 is the specific neat of the brass sample
is the specific neat of the brass sample ![H_g = m_w *c_w * [T_w - T ]](/tpl/images/0692/3585/35bc1.png)
 is the specific heat of water which has a constant value of
is the specific heat of water which has a constant value of 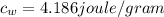
![H_L = H_g \ \equiv m* c_b * [T_i -T] = m_w * c_w * [T - T_w]](/tpl/images/0692/3585/4fcd7.png)
![52.4 * c_b * [95.1 - 17.6] = 150 * 4.186 * [ 17.6 - 15.0]](/tpl/images/0692/3585/31561.png)