Chemistry, 23.06.2020 22:01 haylee1468
For the iodine trichloride molecule: a. Determine the number of valence electrons for each atom in the molecule b. Draw the Lewis Dot structure c. Describe why the molecule is drawn this way (i. e. any extra rules/steps needed?) d. Show the polarity of each bond and for the molecule by drawing in the dipole +à

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Main expenses you plan on making payments on a new car too. you want to spend 15% of your monthly net pay on the car payment, insurance, registration, and taxes combined. what is your monthly car allowance? $149.46 $298.91 $448.37 $597.83
Answers: 3

Chemistry, 22.06.2019 18:30
Which sample at stp has the same number of atoms as 18 liters of ne at stp
Answers: 1

Chemistry, 22.06.2019 18:50
Question 3(multiple choice worth 4 points) (04.04 lc) what does it mean when an element is reduced? it empties a valance shell, reducing its atomic radius. it gains electrons, reducing its overall charge. it increases electronegativity, reducing its ability to bond. it loses electrons, reducing its electron number.
Answers: 1

Chemistry, 22.06.2019 19:30
Astring vibrates with a frequency of 10 hz. why can't a person hear the sound waves produced by the vibrating string, no matter how large the amplitude of the waves? out! this is homework and due tomorrow! you so much!
Answers: 2
You know the right answer?
For the iodine trichloride molecule: a. Determine the number of valence electrons for each atom in t...
Questions

History, 18.10.2019 08:50

Mathematics, 18.10.2019 08:50


Mathematics, 18.10.2019 08:50

Social Studies, 18.10.2019 08:50

Computers and Technology, 18.10.2019 08:50




Physics, 18.10.2019 08:50

Computers and Technology, 18.10.2019 08:50


Mathematics, 18.10.2019 08:50

Mathematics, 18.10.2019 08:50



History, 18.10.2019 08:50

Mathematics, 18.10.2019 08:50

Mathematics, 18.10.2019 08:50

Biology, 18.10.2019 08:50

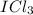 , so the central atom would be "I" and the "Cl" atoms would be placed around "I". See figure 1
, so the central atom would be "I" and the "Cl" atoms would be placed around "I". See figure 1