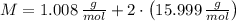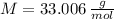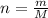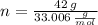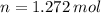Chemistry, 23.06.2020 16:01 leahstubbs
How many moles of \ce{H2O}HX 2 O will be produced from 42.0 \text{ g}42.0 g42, point, 0, start text, space, g, end text of \ce{H2O2}HX 2 OX 2 ?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:40
Silver tarnishes as silver metal reacts with hydrogen sulfide, h2s, in the air. in this reaction, dark silver sulfide, au2s, covers the surface of silver. when silver is polished, this coating of silver sulfide can be removed from the surface. this makes the silver shiny again. enter the coefficients that balance the tarnishing reaction equation. (type 1 for no coefficient.)
Answers: 2

Chemistry, 22.06.2019 05:30
The table describes how some substances were formed substance 19 description formed by boiling pure water formed by combining three hydrogen atoms to every nitrogen atom formed by adding 5 g of sugar to 1 l of water formed by compressing carbon under high pressure based on the given descriptions, which substance is most likely a mixture?
Answers: 1


Chemistry, 22.06.2019 08:30
What method(s) do plants use to obtain nitrogen? select all that apply. absorb it from the atmosphere use bacteria to convert nitrogen to usable form obtain usable nitrogen compounds from the soil absorb nitrogen from water taken in at the roots
Answers: 3
You know the right answer?
How many moles of \ce{H2O}HX 2 O will be produced from 42.0 \text{ g}42.0 g42, point, 0, start text...
Questions

Mathematics, 23.01.2021 19:30

Social Studies, 23.01.2021 19:30




Mathematics, 23.01.2021 19:30


Mathematics, 23.01.2021 19:30


Biology, 23.01.2021 19:30




Chemistry, 23.01.2021 19:30

Computers and Technology, 23.01.2021 19:30


Mathematics, 23.01.2021 19:30


Mathematics, 23.01.2021 19:30

Mathematics, 23.01.2021 19:30

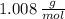 and
and 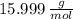 , respectively. A mole is the ratio of current mass of water to molecular weight of water, the latter one is now calculated before computing the amount of moles of water:
, respectively. A mole is the ratio of current mass of water to molecular weight of water, the latter one is now calculated before computing the amount of moles of water: