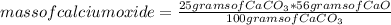Chemistry, 24.06.2020 04:01 torresq6647
How much calcium oxide would be made by the thermal decomposition of 25 grams of calcium carbonate?
CaCO3 -> CaO + CO2

A. 28 grams
B. 12 grams
C. 14 grams
D. 25 grams

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Find the empirical formula of each of the following compounds. given mass or for each element in a sample of the compound 3,611 g ca; 6.389 g c1
Answers: 1

Chemistry, 22.06.2019 14:30
1) describe the physical layout of the ocean floor ? 2) explain how the dumbo octopus swims differently than other octopus species and why this would be an advantage in the aphonic zone . 3) why are the types of organisms that live at each underwater hot vent so dramatically different ?
Answers: 3

Chemistry, 22.06.2019 22:00
Does the number of ions in solution increase, decrease, or remain constant? it continuously decreases. it continuously increases. it decreases at first, then increases. it increases at first, then decreases.
Answers: 3

Chemistry, 23.06.2019 01:30
Which of the following statements is true about energy quantization at the atomic level? electrons in the outermost orbits are the most stable. electrons in all the orbits around the nucleus have the same amount of energy. electrons in the orbit closest to the nucleus have the least amount of energy. electrons absorb or release the same amount of energy independent of the energy levels.
Answers: 1
You know the right answer?
How much calcium oxide would be made by the thermal decomposition of 25 grams of calcium carbonate?...
Questions





Mathematics, 02.07.2019 16:00

Chemistry, 02.07.2019 16:00

Biology, 02.07.2019 16:00

Chemistry, 02.07.2019 16:00


Chemistry, 02.07.2019 16:00




Mathematics, 02.07.2019 16:00


Biology, 02.07.2019 16:00



Chemistry, 02.07.2019 16:00

English, 02.07.2019 16:00