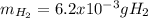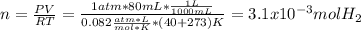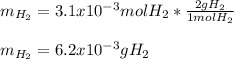Chemistry, 24.06.2020 22:01 keirarae2005
Sometimes in lab we collect the gas formed by a chemical reaction over water. This makes it easy to isolate and measure the amount of gas produced. Suppose H2 the gas evolved by a certain chemical reaction taking place at 40°C is collected over water, using an apparatus something like that in the sketch, and the final volume of gas in the collection tube is measured to be 80ml .
Required:
Calculate the mass of H2 that is in the collection tube. Round your answer to significant digits. You can make any normal and reasonable assumption about the reaction conditions and the nature of the gases.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:10
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1



Chemistry, 22.06.2019 22:30
What relationship exists between an enzyme and a catalyst?
Answers: 1
You know the right answer?
Sometimes in lab we collect the gas formed by a chemical reaction over water. This makes it easy to...
Questions

Mathematics, 20.11.2020 21:50

Biology, 20.11.2020 21:50

History, 20.11.2020 21:50


English, 20.11.2020 21:50

Mathematics, 20.11.2020 21:50




Health, 20.11.2020 21:50

Mathematics, 20.11.2020 21:50


Mathematics, 20.11.2020 21:50


Chemistry, 20.11.2020 21:50

History, 20.11.2020 21:50

Mathematics, 20.11.2020 21:50


Mathematics, 20.11.2020 21:50