
Chemistry, 25.06.2020 07:01 khristaviaaa
Calculate the pH of the acetate buffer CH3COOH + CH3COONa, containing 0.01 of each of the compound. Ka= 1.8×10^-5

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:30
What type(s) of intermolecular forces are expected between ch3ch2cooh molecules? dipole forces, induced dipole forces, hydrogen bonding
Answers: 1

Chemistry, 23.06.2019 01:30
Ascientist conducted an experiment and discovered that certain plants grow faster when given a particular amount of fertilizer. anouther scientist conducted the same experiment and got similar results. which concept does this best illustrate? a) repetition b) replication c) precision d) validity
Answers: 2

Chemistry, 23.06.2019 08:50
What happens after sound waves create vibrations in the ear?
Answers: 1

Chemistry, 23.06.2019 09:50
When scientists are ready to publish the results of their experimentation, why is it important for them to include a description of the procedures they used?
Answers: 1
You know the right answer?
Calculate the
pH of the acetate buffer CH3COOH + CH3COONa, containing 0.01 of each of the compound....
Questions

History, 05.11.2019 04:31



Mathematics, 05.11.2019 04:31

Mathematics, 05.11.2019 04:31

Mathematics, 05.11.2019 04:31

Mathematics, 05.11.2019 04:31

Mathematics, 05.11.2019 04:31




Health, 05.11.2019 04:31




Mathematics, 05.11.2019 04:31


Biology, 05.11.2019 04:31

Mathematics, 05.11.2019 04:31


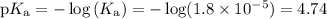
![\begin{array}{rcl}\text{pH} & = & \text{pK}_{\text{a}} + \log \left(\dfrac{[\text{A}^{-}]}{\text{[HA]}}\right )\\\\& = &4.74 +\log \left(\dfrac{0.01}{0.01}\right )\\\\& = & 4.74 + \log1.0 \\& = & 4.74 +0.0\\& = &4.7 \\\end{array}\\\text{The pH is $\large \boxed{\textbf{4.7}}$}](/tpl/images/0693/9328/22ba3.png)


