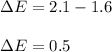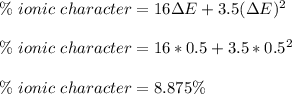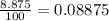Chemistry, 26.06.2020 15:01 jretes8780
The compound gallium phosphide () is a compound semiconductor having mixed ionic and covalent bonding. The electronegativities for and are 1.6 and 2.1 respectively. Calculate the fraction of the bonding that is ionic.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 3

Chemistry, 22.06.2019 15:00
What is the most important factor in determining climates.
Answers: 1

Chemistry, 22.06.2019 22:30
Akno3 solution containing 51 g of kno3 per 100.0 g of water is cooled from 40 ∘c to 0 ∘c. what will happen during cooling?
Answers: 3

Chemistry, 22.06.2019 22:40
Covalent bonds generally form when the bonded elements have a difference in electronegativity less than 1.5. subtract the electronegativities for the following pairs of elements and predict whether they form a covalent bond. electronegativity difference of c and c: ionic covalent electronegativity difference of mg and cl: ionic covalent
Answers: 1
You know the right answer?
The compound gallium phosphide () is a compound semiconductor having mixed ionic and covalent bondin...
Questions


Mathematics, 26.10.2020 18:20





Biology, 26.10.2020 18:20


Mathematics, 26.10.2020 18:20

Biology, 26.10.2020 18:20




Chemistry, 26.10.2020 18:20


Mathematics, 26.10.2020 18:20



Mathematics, 26.10.2020 18:20

Arts, 26.10.2020 18:20